

 Image of Gas
Image of Gas
Introduction:
Gas is a state of matter characterized by its ability to expand to fill any container it occupies. Comprising a variety of elements and compounds, gases play a crucial role in our daily lives, from providing energy to supporting various industrial processes. In this article, we will explore the properties, uses, and environmental impact of gases.
Properties of Gases:
Gases differ from solids and liquids in that they have no fixed shape or volume. Instead, they take the shape of their container and expand to fill the available space. The behavior of gases is governed by principles like Boyle’s Law, Charles’s Law, and Avogadro’s Law. These laws describe how changes in pressure, temperature, and volume affect the behavior of gases.
Common Gases and their Uses:
Oxygen (O2) is essential for various biological, industrial, and medical processes. Here are some key uses of oxygen:
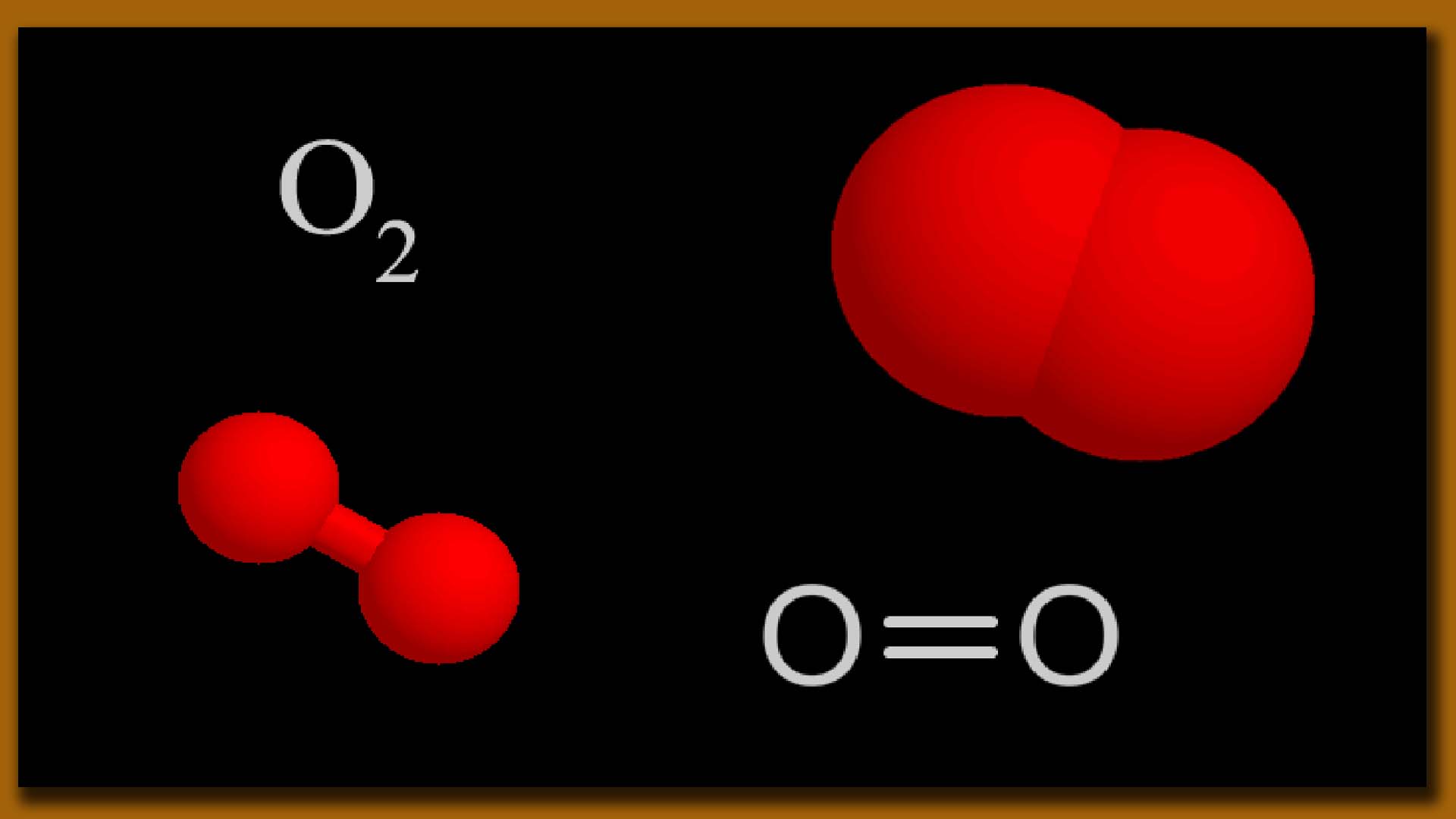
Oxygen molecule formation Picture
i. Cellular Respiration: In living organisms, oxygen is crucial for cellular respiration, a process where cells use oxygen to generate energy (in the form of ATP) by oxidizing glucose.
ii. Metallurgy: Oxygen is often used in metallurgical processes, such as the production of steel. It helps in the combustion of impurities and facilitates the formation of oxides.
iii. Oxidation Processes: Many chemical reactions involve the oxidation of substances, and oxygen is a key element in these processes. For example, combustion reactions, where substances react with oxygen to produce heat and light.
iv. Medical Applications: Oxygen is commonly used in medical settings for respiratory support. It is administered to patients who have difficulty breathing, such as those with respiratory illnesses, during surgery, or in emergency situations.
v. Oxygen Therapy: In addition to medical emergencies, oxygen therapy is employed for chronic respiratory conditions like chronic obstructive pulmonary disease (COPD) to improve the patient’s oxygen levels.
vi. Water Treatment: In water treatment processes, oxygen is used to facilitate the biological treatment of wastewater. It supports the growth of aerobic bacteria, which helps break down organic pollutants.
vii. Rocket Propulsion: Liquid oxygen is a common oxidizer used in rocket engines. It provides the oxygen necessary for the combustion of rocket propellants, allowing for the generation of thrust.
viii. Welding and Cutting: Oxygen is used in oxy-fuel welding and cutting processes. When combined with acetylene or other fuels, it supports high-temperature combustion for welding and metal cutting applications.
ix. Chemical Synthesis: Oxygen is a key element in various chemical synthesis processes, including the production of chemicals, pharmaceuticals, and other industrial compounds.
x. Food and Beverage Industry: In food packaging, oxygen is often removed to extend the shelf life of products. It can also be used in controlled environments to influence the fermentation process in certain food and beverage production.
These are just a few examples, and oxygen plays a vital role in numerous other industrial, biological, and scientific applications.
A lightweight and highly flammable gas, hydrogen has applications in industries such as petroleum refining, metal production, and the production of ammonia. It is also considered a potential clean energy carrier when used in fuel cells. Some of the key applications of hydrogen gas include:
A Molecule of Hydrogen (H2) Gas
1.Energy Production:
• Fuel Cells: Hydrogen can be used as a fuel in fuel cells to generate electricity through an electrochemical process, with water and heat being the only byproducts. This makes fuel cells a clean and efficient energy conversion technology.
• Hydrogen Combustion: Hydrogen can also be burned as a fuel in internal combustion engines or turbines to produce mechanical or electrical energy.
2. Industrial Processes:
• Chemical Production: Hydrogen is a crucial feedstock for the production of various chemicals, including ammonia, methanol, and synthetic fuels.
• Oil Refining: Hydrogen is used in oil refining processes, such as hydrocracking and desulfurization, to improve the quality of refined products.
• Metal Production: It is employed in the reduction of metals from their ores, such as in the production of steel and other alloys.
3. Electronics and Semiconductor Manufacturing:
• Hydrogen is used as a reducing agent and carrier gas in the production of semiconductors and electronic components.
4. Food Industry:
• In the food industry, hydrogen is used for hydrogenation processes, which is crucial in the production of fats and oils.
5. Aerospace and Rocket Propulsion:
•Hydrogen is used as a fuel in rocket engines due to its high energy content and efficiency.
6. Balloon and Airship Inflation:
• Historically, hydrogen was used to inflate balloons and airships due to its low density. However, this application has become less common due to safety concerns, as hydrogen is flammable.
7. Power Generation:
• Hydrogen can be burned in conventional power plants to generate electricity.
8. Hydrogen Storage and Transportation:
• Hydrogen can be used as an energy carrier and stored for later use. Efforts are ongoing to develop efficient methods for hydrogen storage and transportation.
9. Medical Applications:
• Hydrogen is used in various medical applications, such as in the production of certain pharmaceuticals and as a cooling agent in magnetic resonance imaging (MRI) systems.
10. Laboratory and Research:
•Hydrogen is used in laboratories for various experiments and as a carrier gas in chromatography.
It’s worth noting that while hydrogen has numerous applications, challenges such as production methods, storage, and distribution infrastructure need to be addressed to realize its full potential as a clean and sustainable energy carrier.
Carbon dioxide (CO2) gas has various uses in different industries and applications. Some of the common uses of carbon dioxide include:
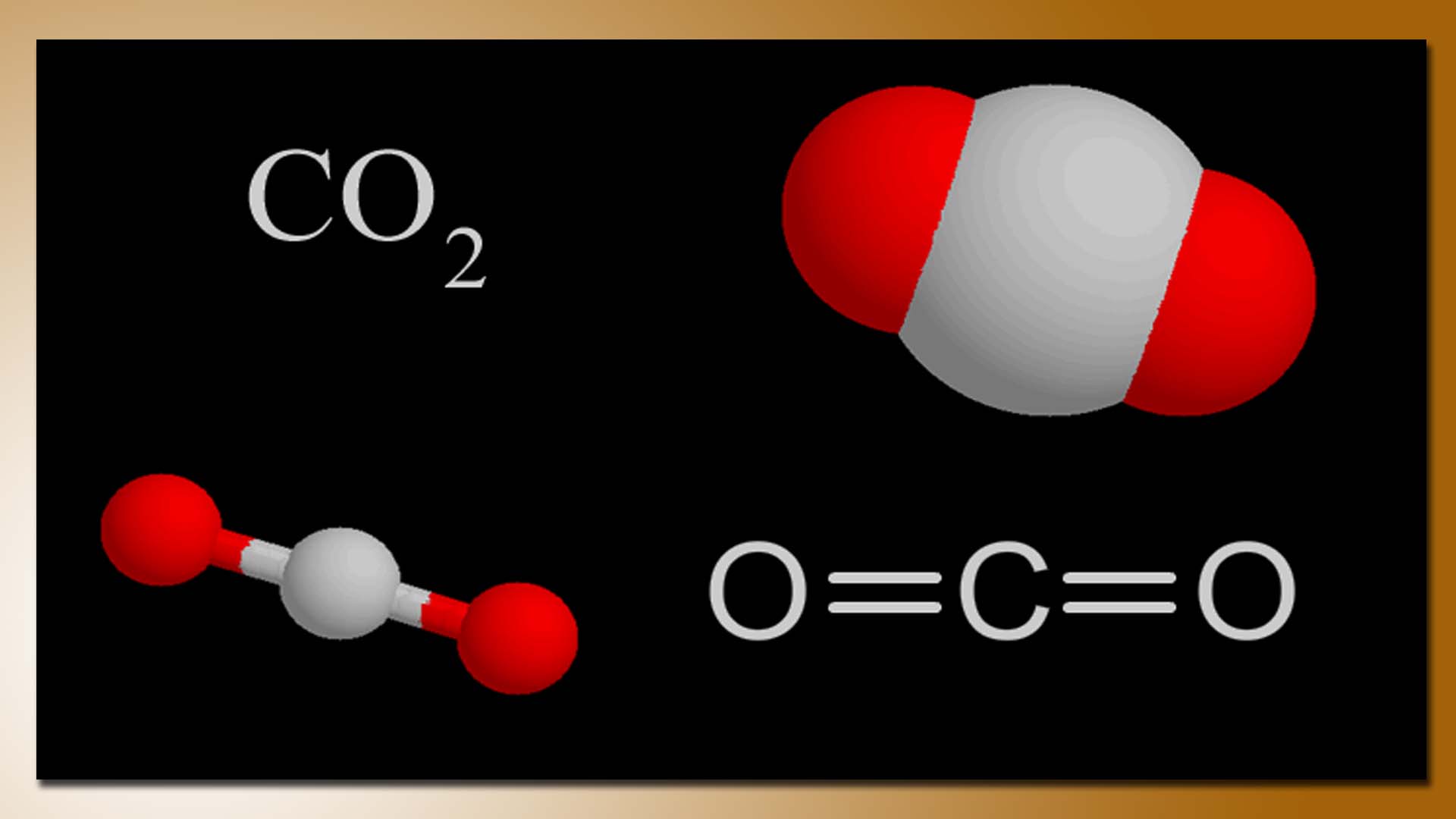
A Molecule of Carbondi Oxide Gas
1. Carbonated Beverages:
• CO2 is widely used in the beverage industry to carbonate soft drinks, beer, and other carbonated beverages. It adds effervescence and enhances the taste and mouthfeel of these drinks.
2. Food Processing:
• CO2 is used in food processing as a cooling agent and to control pH levels. It is commonly employed in freezing and chilling applications for food preservation.
3. Fire Extinguishers:
• Carbon dioxide is an effective fire suppressant. It is used in fire extinguishers to displace oxygen, thereby extinguishing the fire by removing the oxygen necessary for combustion.
4. Medical Applications:
• In the medical field, carbon dioxide is used in various applications, such as in respiratory therapies and as a component in the calibration of medical equipment.
5. Industrial Processes:
• CO2 is utilized in various industrial processes, including welding, where it is used as a shielding gas to prevent oxidation during the welding process.
6. Oil Recovery:
• In enhanced oil recovery (EOR) methods, carbon dioxide is injected into oil reservoirs to increase oil extraction efficiency.
7. Greenhouses:
• Carbon dioxide is often supplied to greenhouses to enhance plant growth through a process known as carbon dioxide enrichment. Increased CO2 levels can stimulate photosynthesis in plants.
8. Chemical Production:
• CO2 is used in the production of chemicals, including urea and salicylic acid. It is also employed as a raw material in the synthesis of various organic compounds.
9. Pharmaceuticals:
• Carbon dioxide is used in the pharmaceutical industry for processes such as the extraction of drugs and as a cryogenic agent for the storage of biological materials.
10. Water Treatment:
• CO2 is used in water treatment processes to control pH levels and alkalinity in both industrial and municipal water treatment facilities.
11. Dry Ice Production:
• Solid carbon dioxide, known as dry ice, is used for cooling and freezing applications, including transportation of perishable goods and in theatrical productions for creating smoke or fog effects.
12. Refrigeration:
• In certain refrigeration systems, carbon dioxide is used as a refrigerant, especially in environmentally friendly and energy-efficient applications.
It’s important to note that while carbon dioxide has various industrial applications, its increasing concentration in the Earth’s atmosphere due to human activities, such as burning fossil fuels, is a major contributor to climate change and global warming. Efforts to reduce carbon dioxide emissions are critical in mitigating the impacts of climate change.
Methane (CH4) is a versatile hydrocarbon gas with various uses across different industries and applications. Some of the primary uses of methane include:
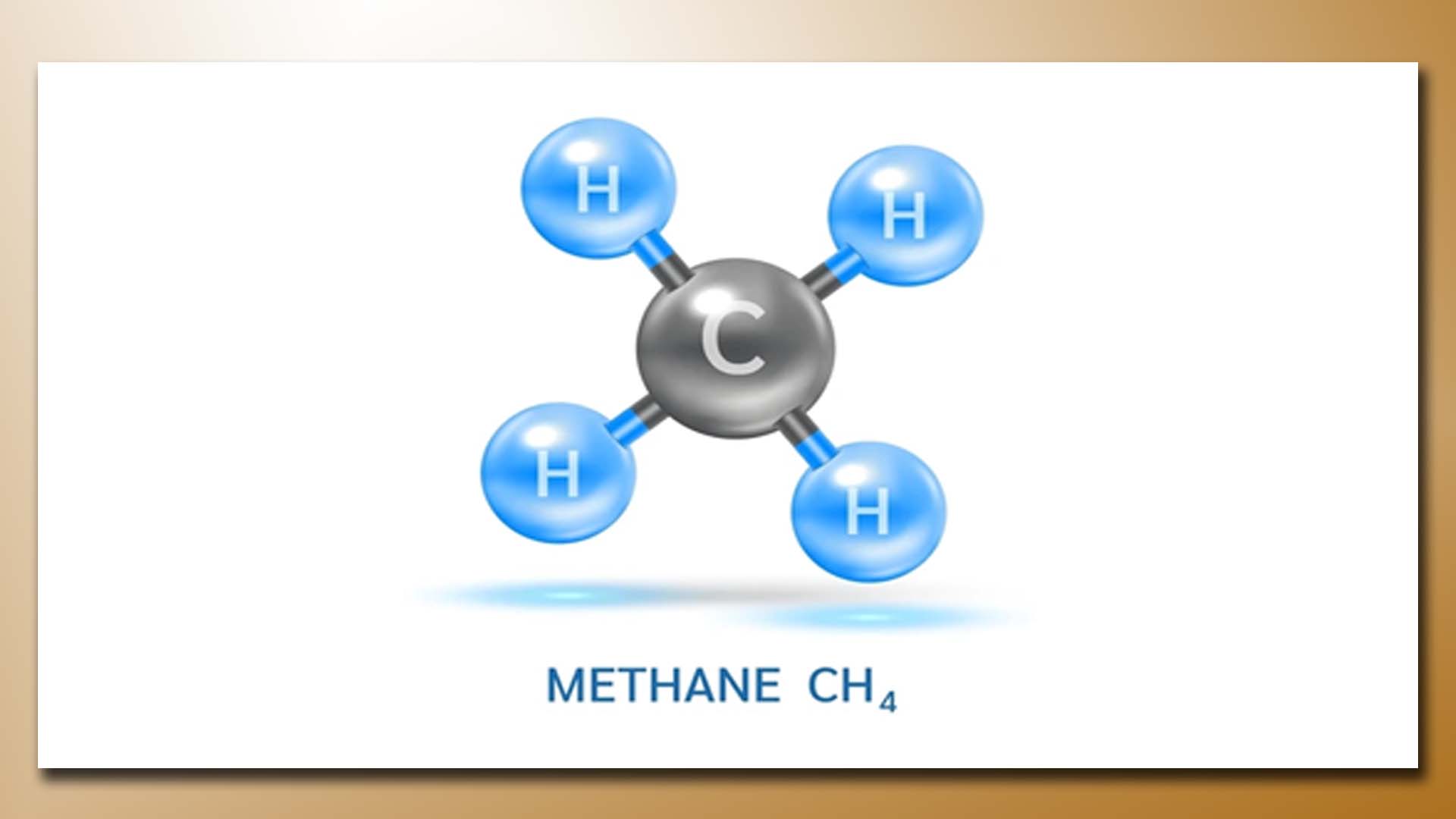
A Molecule of Methane (CH4) Gas
1. Fuel Source:
• Natural Gas: Methane is the primary component of natural gas, which is a widely used fuel for heating, cooking, and electricity generation. It burns more cleanly than many other fossil fuels, emitting lower levels of carbon dioxide and other pollutants.
2. Electricity Generation:
• Power Plants: Methane can be used as a fuel for power plants to generate electricity. Combined cycle power plants, in particular, can achieve high efficiency when using natural gas.
3. Transportation:
• Vehicle Fuel: Methane can be used as a fuel for vehicles, either directly as compressed natural gas (CNG) or as liquefied natural gas (LNG). Methane-powered vehicles produce lower emissions compared to traditional gasoline or diesel vehicles.
4. Industrial Processes:
• Chemical Feedstock: Methane is a precursor for the production of various chemicals, such as hydrogen, methanol, and ammonia, which are essential in the manufacturing of a wide range of products.
5. Heating and Cooking:
• Residential and Commercial Use: Methane is commonly used for heating homes and buildings as well as for cooking. It is a convenient and efficient source of energy for these applications.
6. Anaerobic Digestion:
• Biogas Production: Methane is produced naturally through anaerobic digestion of organic matter, such as sewage, agricultural waste, and landfills. The resulting biogas, which contains methane, can be captured and used as a renewable energy source.
7. Greenhouse Gas:
• While methane is a potent greenhouse gas itself, capturing and utilizing it can help reduce its impact on the environment. Methane emissions from sources like landfills and wastewater treatment can be mitigated by capturing and using the gas for energy.
8. Enhanced Oil Recovery (EOR):
• Oil and Gas Industry: Methane can be used in enhanced oil recovery processes, where it is injected into oil reservoirs to help recover additional oil.
9. Chemical and Pharmaceutical Industries:
• Synthesis of Organic Compounds: Methane serves as a feedstock for the synthesis of various organic compounds used in the chemical and pharmaceutical industries.
10. Research and Laboratories:
• Calibration Gas: Methane is used as a calibration gas in laboratories for analytical instruments and gas detectors.
It’s important to note that while methane has many valuable applications, its role as a greenhouse gas and its contribution to climate change have raised concerns. Efforts to capture and reduce methane emissions are ongoing to address environmental impacts.
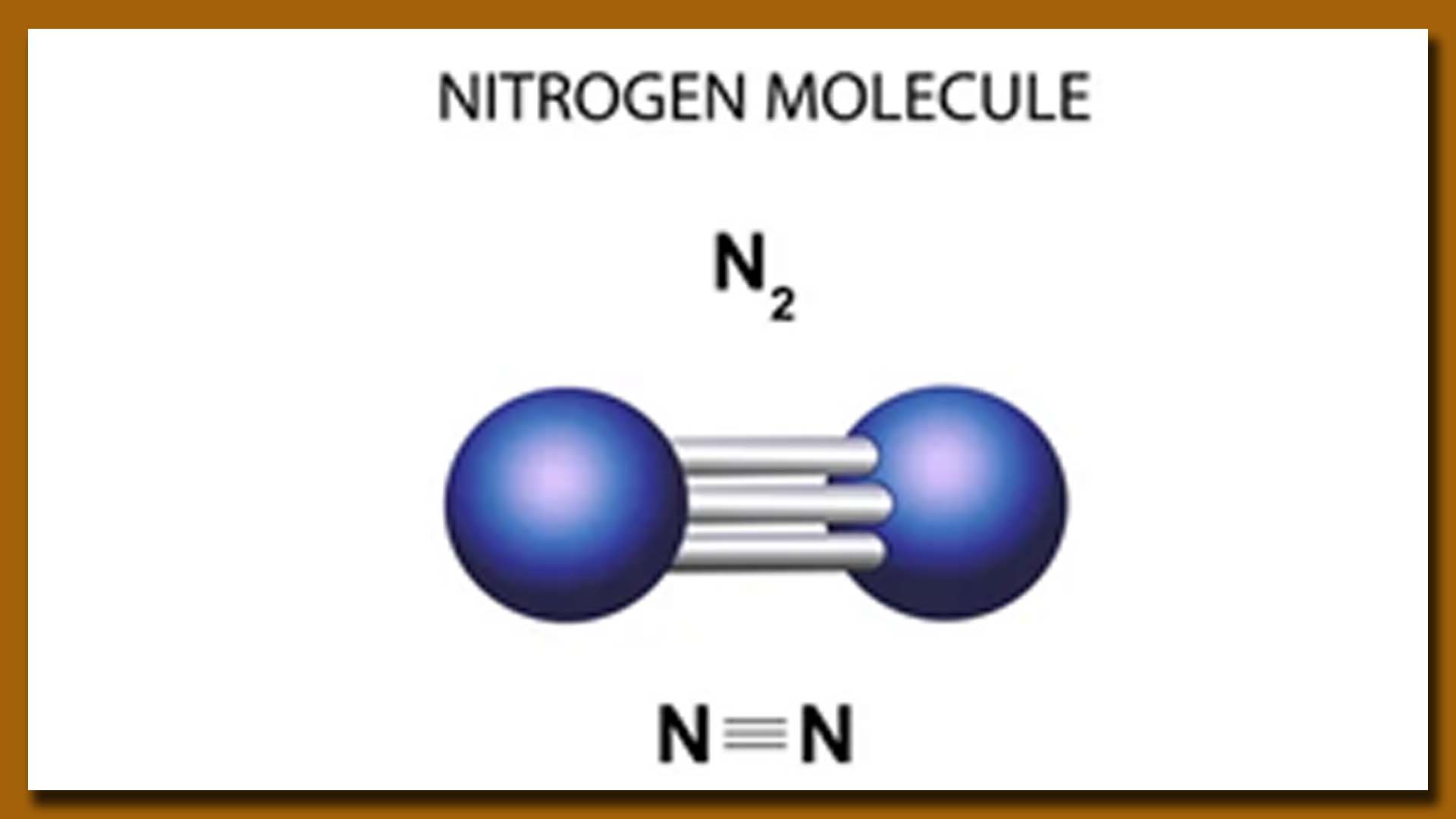
Nitrogen (N2) is a versatile element with various applications across different industries. Some of the key uses of nitrogen include:
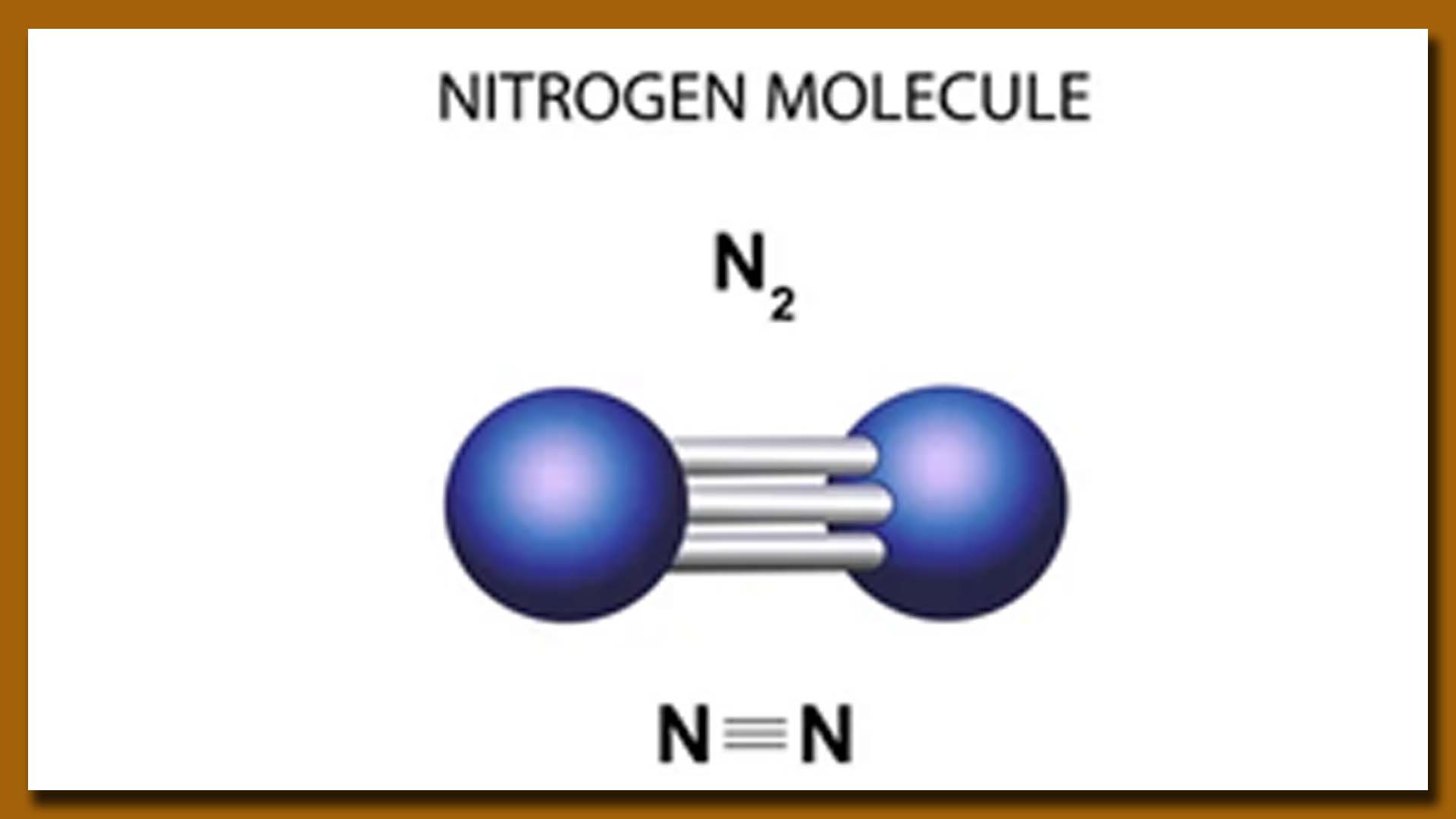
A Molecule of Hydrogen (H2) Gas
1. Chemical Industry:
• Nitrogen is used as a blanketing gas to prevent the oxidation and degradation of sensitive materials during production and storage.
• It is a key component in the synthesis of ammonia, which is used to produce fertilizers, explosives, and various chemicals.
2. Food Industry:
• Nitrogen is employed in the food packaging industry to displace oxygen and extend the shelf life of perishable goods. This process is known as Modified Atmosphere Packaging (MAP).
• It is also used in the freezing and chilling of food products to prevent freezer burn and maintain product quality.
3. Metallurgy:
• Nitrogen is used as a cooling and purging gas in the heat treatment of metals, preventing oxidation and maintaining the desired metallurgical properties.
• In the production of stainless steel, nitrogen is used to enhance the corrosion resistance of the material.
4. Electronics Industry:
• Nitrogen is utilized in the electronics industry during the manufacturing of semiconductors and other electronic components. It helps create an inert environment to prevent oxidation during certain processes.
5. Medical Applications:
• Liquid nitrogen is used in cryopreservation to preserve biological samples, such as cells, tissues, and sperm, at extremely low temperatures.
• Nitrogen gas is used in medical laboratories for sample preservation and storage.
6. Welding and Metal Cutting:
• Nitrogen is employed in some welding and cutting processes to create an inert atmosphere, preventing oxidation and ensuring a clean weld or cut.
7. Agriculture:
• As a component of fertilizers, nitrogen is essential for plant growth. It is often applied in the form of ammonia, urea, or other nitrogen-containing compounds to improve soil fertility.
8. Oil and Gas Industry:
• Nitrogen is used for various purposes in the oil and gas industry, including enhanced oil recovery, well stimulation, and purging pipelines and equipment to prevent corrosion.
9. Pharmaceutical Industry:
• Nitrogen is used in pharmaceutical manufacturing for processes such as freeze-drying and purging equipment to maintain a controlled and inert environment.
10. Laboratory and Research:
• Nitrogen is commonly used in laboratories for various analytical and research applications, such as gas chromatography and as a carrier gas in mass spectrometry.
These are just a few examples, and the versatility of nitrogen makes it an essential element in numerous industrial processes and applications.
While gases play a crucial role in various industrial processes and our daily lives, their impact on the environment is a growing concern. The combustion of fossil fuels releases carbon dioxide, contributing to global warming. Methane, another greenhouse gas, is released during natural gas production and distribution. Efforts to reduce emissions and transition to cleaner energy sources are underway to mitigate these environmental impacts.
The environmental impact of gas, particularly in the context of energy and industry, depends on the type of gas and how it is used. Here, I’ll discuss the environmental impact of three main types of gases: carbon dioxide (CO2), methane (CH4), and nitrogen oxides (NOx).
Efforts to mitigate the environmental impact of gases include transitioning to cleaner energy sources, improving energy efficiency, capturing and storing emissions, and implementing regulations to limit emissions from various sources. The shift towards renewable energy, electrification of transportation, and sustainable land use practices are key strategies in reducing the overall impact of gases on the environment.
Conclusion:
Understanding the properties, uses, and environmental impact of gases is crucial for responsible and sustainable practices. As society continues to evolve, finding cleaner alternatives and implementing more efficient technologies will be essential to minimize the environmental footprint associated with gas usage. From energy production to daily conveniences, gases are a fundamental aspect of modern life that requires thoughtful consideration for a more sustainable future.
Formula of Common Gases:
Here are some frequently asked questions (FAQs) about natural gas:
1. What is natural gas?
Ans: Natural gas is a fossil fuel that primarily consists of methane. It is formed deep beneath the Earth’s surface over millions of years from the remains of plants and animals. It is extracted from underground reservoirs and used as a source of energy.
2. How is natural gas formed?
Ans: Natural gas is formed through the decomposition of organic matter, such as plants and animals, under high pressure and heat over millions of years. This process occurs in the Earth’s crust, resulting in the creation of hydrocarbons, including methane.
3. What are the main components of natural gas?
Ans: The primary component of natural gas is methane (CH4), but it can also contain small amounts of other hydrocarbons, such as ethane, propane, and butane, as well as impurities like carbon dioxide, nitrogen, and sulfur compounds.
4. How is natural gas extracted?
Ans: Natural gas is extracted from underground reservoirs through drilling wells. The most common methods include traditional vertical drilling and hydraulic fracturing (fracking) for unconventional sources like shale gas
5. What are the main uses of natural gas?
Ans: Natural gas is used for various purposes, including heating homes and buildings, generating electricity, fueling vehicles, and as a raw material in the production of chemicals and fertilizers.
6. Is natural gas considered a clean energy source?
Ans: While natural gas is considered cleaner than some other fossil fuels, such as coal and oil, burning it still produces carbon dioxide (CO2), a greenhouse gas. The extraction process, especially in hydraulic fracturing, has environmental concerns.
7. How is natural gas transported?
Ans: Natural gas is transported through pipelines, which crisscross countries and continents. It can also be transported in its liquefied form (LNG) by specialized ships, especially for international trade.
8. What is the difference between natural gas and propane?
Ans: Both natural gas and propane are hydrocarbons, but natural gas is primarily methane, while propane is a liquefied petroleum gas (LPG) consisting of propane molecules. Propane is stored and transported in a liquid state but becomes a gas when released.
9. Can natural gas be used as a vehicle fuel?
Ans: Yes, natural gas is used as a vehicle fuel in compressed natural gas (CNG) or liquefied natural gas (LNG) form. It is considered a cleaner alternative to traditional gasoline or diesel.
10. What are the environmental concerns associated with natural gas?
Ans: Environmental concerns include methane leakage during extraction and transportation, which is a potent greenhouse gas. Additionally, the extraction method of hydraulic fracturing has raised concerns about water contamination and other environmental impacts.
11. Is there a difference between conventional and unconventional natural gas?
Ans: Conventional natural gas is found in easily accessible reservoirs, while unconventional natural gas, such as shale gas, tight gas, and coalbed methane, requires advanced extraction methods like hydraulic fracturing.
These FAQs cover some basic aspects of natural gas, but it’s important to note that specific details may vary based on regional practices and regulations.